
Developmental Biology Lab

實驗室研究方向-- CTP合成酶線狀結構如何影響癌症的形成和影響果蠅的發育-白麗美老師
我的研究聚焦於探討 CTP 合成酶線狀結構(Cytidine Triphosphate Synthase Filament,簡稱 CTPS filament)在癌症形成與果蠅(Drosophila melanogaster)發育中的功能與調控機制。
CTP 合成酶(CTPS)是細胞生長不可或缺的關鍵酵素,負責催化胞嘧啶三磷酸(CTP)的合成。CTP 不僅是 DNA 與 RNA 合成的基本單元,亦在脂質生成與醣化反應中扮演重要角色。腫瘤形成通常源自癌細胞異常增生,並伴隨細胞生長相關基因(如致癌基因)的變異;同時,癌細胞會重新塑造其代謝途徑,高度依賴葡萄糖與麩醯胺酸(glutamine)等營養來源,以支撐快速增殖。然而,腫瘤微環境中常因資源消耗而面臨麩醯胺酸缺乏的營養壓力。

Masaru Goto et.al.,Structure,2004

我們的研究發現,在此營養逆境下,癌細胞可透過形成 CTPS 線狀結構 來調節代謝反應,進而提升其存活與適應能力 。我們進一步證實 CTPS 線狀結構會聚集於細胞骨架上 ,且此結構的形成可促進腫瘤生長 ,顯示代謝酵素的空間組織化在癌症進程中具有關鍵功能。
此外,我們在果蠅生殖細胞(卵室)中觀察到 CTPS 形成高度保守的線狀結構,並證實其聚集狀態可調控酵素活性,以滿足 DNA 快速複製的需求,凸顯生化反應區域化對細胞生理調控的深遠影響 。


本實驗室整合遺傳學、蛋白質體學、代謝體學與基因體學等多層次研究策略,深入解析癌細胞在營養壓力下的逆境生存機制,並揭示區域化生化反應調控在果蠅發育中的關鍵角色。同時,我們亦致力於探討蛋白質轉譯後修飾(如泛素化與甲基化)如何調節 CTPS 聚集,從分子層級闡明其生理與病理意義。
研究重點
本實驗室透過整合 基因體學、代謝體學與蛋白質體學分析,系統性解析癌細胞在營養壓力下的適應機制,並深入探討其如何動態調控 CTPS 線狀結構 以維持代謝平衡與細胞存活。我們進一步關注此類逆境生存策略是否可被藥理性干擾,作為發展新型癌症治療標靶的潛在切入點。
本實驗室的研究重點包括:
-
探討 CTPS 線狀結構於果蠅生殖細胞(卵室與精子)中的形成機制,以及其與細胞週期調控與細胞分化之間的關聯。
-
解析癌細胞在麩醯胺酸缺乏的營養逆境中調控 CTPS 線狀結構的分子機制,並評估其對細胞存活與腫瘤生長的影響。
-
評估 CTPS 線狀結構形成與穩定機制作為標靶藥物的可行性,以探索干擾代謝酵素區域化作為抗癌治療的新策略。
透過上述研究,我們旨在闡明 CTPS線狀結構在細胞生理與腫瘤形成中的功能與調控機制,並為新型代謝導向之抗癌療法奠定理論基礎。
The Research Direction – How the Linear Structure of CTP Synthase Affects Cancer Formation and Influences the Development of Drosophila,
Li-Mei Pai PhD.
Our laboratory focuses on elucidating the roles and regulatory mechanisms of cytidine triphosphate synthase (CTPS) filament formation in cancer development and Drosophila melanogaster development.
CTP synthase (CTPS) is an essential metabolic enzyme required for cell growth, catalyzing the synthesis of cytidine triphosphate (CTP). CTP is a fundamental building block for DNA and RNA synthesis and also plays critical roles in lipid biosynthesis and glycosylation. To sustain rapid proliferation, cancer cells reprogram their metabolic pathways and become highly dependent on nutrients such as glucose and glutamine. However, nutrient depletion—particularly glutamine limitation—is a common stress within the tumor microenvironment.

Masaru Goto et.al.,Structure,2004
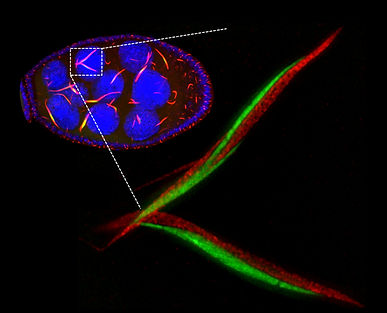
Our research demonstrates that under such nutrient stress conditions, cancer cells adapt by forming CTPS filaments, which modulate metabolic activity and promote cell survival. We further show that CTPS filaments associate with the cytoskeleton, and that filament formation enhances tumor growth, highlighting the importance of metabolic enzyme spatial organization in cancer progression.
In addition to cancer biology, we investigate the physiological roles of CTPS filament formation in vivo using Drosophila. We observe highly conserved CTPS filaments in germline cells (egg chambers) and demonstrate that CTPS assembly regulates enzymatic activity to meet the high demand for rapid DNA replication. These findings underscore the significance of spatially organized biochemical reactions in cellular physiology and developmental processes.


By integrating genetics, proteomics, metabolomics, and genomics, our laboratory systematically dissects the adaptive mechanisms employed by cancer cells under nutrient stress. We also investigate how post-translational modifications, such as ubiquitination and methylation, regulate CTPS filament assembly, providing mechanistic insights into their roles in cancer and development.
Research objectives
Our laboratory integrates genomics, metabolomics, and proteomics to systematically dissect the adaptive mechanisms employed by cancer cells under nutrient stress. We focus on understanding how cancer cells dynamically regulate CTPS filament formation to maintain metabolic homeostasis and promote survival. In parallel, we aim to explore whether this stress-adaptive strategy can be pharmacologically targeted, thereby opening new avenues for cancer therapy.
Our major research interests include:
-
Elucidating the mechanisms of CTPS filament formation in Drosophila germ cells (egg chambers and sperm), and determining their roles in cell cycle regulation and cellular differentiation.
-
Defining the molecular mechanisms by which cancer cells regulate CTPS filament dynamics under glutamine-deprived conditions, and assessing their impact on cell survival and tumor growth.
-
Evaluating the therapeutic potential of targeting CTPS filament assembly and stability, with the goal of developing novel metabolism-based anticancer strategies.
Through these studies, we aim to define the functional and regulatory roles of CTPS filament formation in cellular physiology and tumorigenesis, and to provide a conceptual framework for the development of next-generation anticancer therapies.